The color of a light source can be unravelled into it’s principal components using a diffraction grating.
Each separate color (wavelength) is bent to a different position, resulting in a rainbow like image.
To best separate each color, a photo is taken of the light source behind a narrow slit. The resulting spectrum is an infite numbers of copies of that slit, spread out into a spectrum.
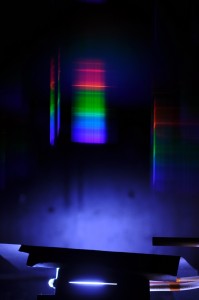
Setup, showing the slit below, with the spark gap behind it and the diffraction spectrum floating above in air
A Cokin P040 diffraction grating (240 lines per mm) was simply placed in front of a DSLR lense (5 Euros only, I was lucky, 40 Euros is a typical price), resulting in the following image:
Since air consists of 80% Nitrogen, 20% Oxygen and a small fraction of Argon, it’s spectrum should be a mix of the spectra of these atoms (some simulated spectra here). But when I look at this spectrum, I find it hard to match them. Also it lacks any hint of yellow tones, to me it is just RG&B.
For comparison I have also imaged my Nikon SB-600. I would expect a spectrum similar to Xenon, but also that does not really look like it.
For reference, these are the spectra from Joachim Koppen’s website (http://astro.u-strasbg.fr/~koppen/).
Finally an attempt to match the spectra of the air-gap flash to Oxygen and Nitrogen. It seems that the spectrum mainly consists of Nitrogen (which was to be expected). The simulated spectra of Oxygen en Nitrogen were blurred to match the resolution of the grating/slit photo.



Very cool technique!
I’ve been wondering how complete the color spectrum is with an air gap flash. I know there are some light sources like sodium vapor lamps that are completely missing some areas of the spectrum and can’t be color-corrected with a simple white balance adjustment.
There doesn’t seem to be much yellow spectrum in your test, though it’s not a gap – do you think it’s just an artifact of your diffraction grating, or is really missing? Have you tested a color target like the ColorPassport to see if it’s possible to calibrate this light source?
Very interesting remark! I had the same observation and did a simple check: look through the viewfinder to see the spectrum “live” when firing the flash. Even though the spectrum lasts on one microsecond, you can still have a look. And the spectrum clearly showed a range of yellow tones, like you see in the simulated nitrogen spectrum. Actually, it looked 10x more beautiful live, than on the final photo. I have used “sunny” white balance to make sure that this was not a white balance effect. I suspect the bright orange line in the nitrogen spectrum is actually yellow in real life.
My conclusion is, that an RGB sensor is not really capable of capturing monochromatic yellow. That color falls between the red and green sensors.
Looking on the internet I found that an range of different filter arrays than RGB exist:
http://en.wikipedia.org/wiki/Color_filter_array
maybe some of them do a better job at preserving yellow.